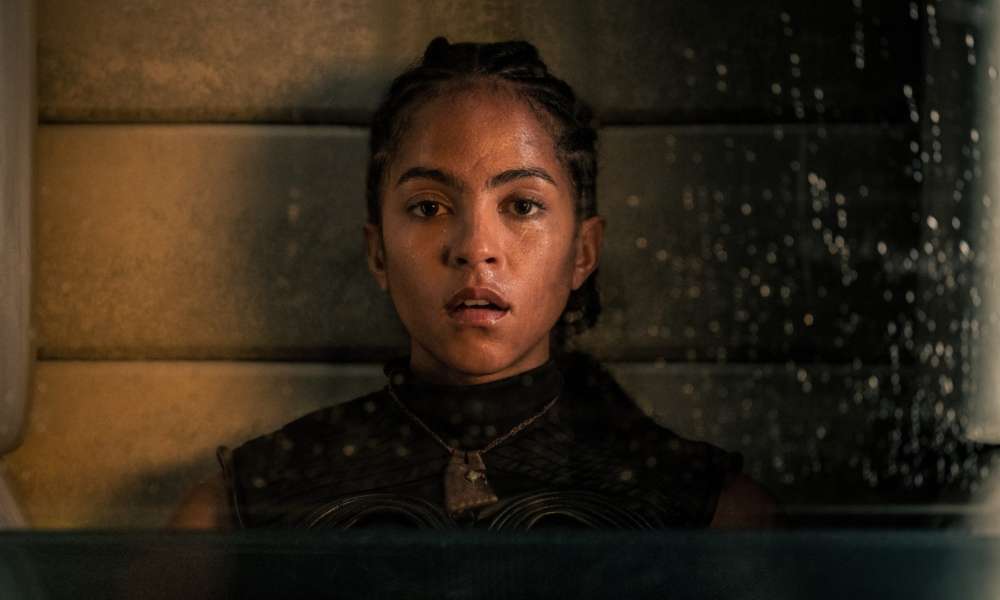If you’re only watching Foundation for space explosions and Lee Pace outfits, you’re missing the real revolution. In this breakdown of Foundation Season 2 explained, we explore how the show took everything wild from Season 1, like clone drama, psychic ...
This Foundation Season 2 Episode 1 recap dives into “In Seldon’s Shadow,” where Cleons crack, assassins strike, and Hari pulls strings from digital purgatory. We’re back in the galaxy far, far mathier, and Foundation Season 2 wastes no time shaking ...
Thank you FANdation, We are back with another recap of our favorite “I’m not Crying” show Foundation Season 2 Episode 10. I’m your host, Anthony, and I will do my best to be your guide in this far-out show as ...
Thank you #FANdation, we are back with another FOUNDATION Season 2 theory! In this video, we’re going to discuss the increased power levels of Gaal Dornick. We see Gaal’s emerging powers throughout Season 2 of Foundation, especially in Episode 9. In ...
Thank you #FANdation, we are back with another FOUNDATION Season 2 theory! In this video, we’re going to discuss the recent details ending to episode 9 and the details that might be hiding IN PLAIN SIGHT! In the boundless realm ...
Thank you FANdation, We are back with another video on our favorite “blink and you miss it” show FOUNDATION Season 2. In this video, we’re going to discuss the recent details about the style of storytelling in this season of ...
Thank you FANdation, We are back with another recap of our favorite “I don’t even know who’s really in charge anymore” show Foundation Season 2 Episode 9. I’m your host, Anthony, and I will do my best to be your guide ...
Thank you FANdation for checking out our FOUNDATION Season 2 Theory. In this video, we’re going to discuss the recent confirmation that the ever-lovable Beki is NOT the secret ingredient for the first Foundation’s Whisper Ships. One of my favorite ...
Thank you FANdation, We are back with another recap of our favorite “I don’t even know who’s really in charge anymore” show Foundation Season 2 Episode 8. I’m your host, Anthony, and I will do my best to be your guide ...
Thank you FANdation, We are back with another recap of our favorite “I don’t even know who’s really in charge anymore” show Foundation Season 2 Episode 6. I’m your host, Anthony, and I will do my best to be your guide ...
Thank you FANdation, We are back with another recap of our favorite “I don’t even know who’s really in charge anymore” show Foundation Season 2 Episode 5. We’re actually picking up where Episode 3 ended where we finally catch up ...
Thank you FANdation, We are back with another recap of our favorite “The future is effed up” show Foundation Season 2 Episode 2. We’re picking up where Episode 1 ended with Gaal and Hari Seldon preparing to have a reckoning ...